What Is Differentially Protein Expression several units call amino acids. Our body needs dietary proteins to provide amino acids to promote the growth and maintenance of its cells and tissues. Our dietary protein needs to changed throughout life. The European Food Safety Authority (EFSA) recommends that adults consume at least 0.83 grams of protein per kilogram of body weight per day (for example, for a 70 kg adult, 58 grams of protein per day). Protein-based protein quality and digestibility vary, but if most people’s total protein meets their needs, it is generally not a concern for most people. We should try to consume protein from various sources, which is good for our health as well as the planet.
Differential Protein Expression Analysis
Each protein has specific characteristics. These properties depend on the number and specific sequence of amino acids in the protein molecule. These characteristics also depend on the size of the protein. This gives the protein its final shape. These are the four organizational levels of proteins:

1.Primary Structure of Proteins
The protein structure showing the number and sequence of amino acids called the primary structure of the protein.
When amino acids arranged linearly in a polypeptide chain, its called the primary structure of the protein.
Proteins also contain disulfide bonds (S-S). Insulin and hemoglobin protein are examples of primary structures.
Classification of Protein
A) amino acid sequence in protein
Insulin molecular sequence
Sanger first determines the sequence of the protein molecule. Sanger has engaged in insulin research for ten years. They found that insulin contains 51 amino acids. It has two amino acid chains. A chain contains 21 amino acids. The other series contains 30 amino acids. The two chains connected by disulfide bonds (bonds).
Sequence of hemoglobin molecule
Hemoglobin discovered by Ingram (scientist). Hemoglobin contains 574 amino acids. These amino acids form a chain of four amino acids. It has two alpha and two beta chains. Each α chain contains 141 amino acids, and each 146 chain contains 146 amino acids.
B) Size of protein molecules
The size of the protein depends on the type and number of amino acids in the protein.
C) Specific arrangement of amino acids
There are 1,000 types of proteins in the human body. Each protein has a unique and unique arrangement of 20 types of amino acids. DNA has a nucleotide sequence. The nucleotide sequence determines the sequence of amino acids in the protein molecule.
The arrangement of amino acids highly specialized for the normal function of proteins. If not a single amino acid is in its normal state, then the protein will not be able to perform its normal function. The best example is the human sickle cell hemoglobin. In this case, one amino acid molecule replaced by another amino acid. Hemoglobin cannot carry enough oxygen. May cause death.
2. Secondary structure of proteins
The protein structure formed by twisting and twisting the polypeptide chain called the secondary structure of the protein. Protein molecules are usually not flat. There are several ways to coil:
The hypotenuse (eg spring-like) curl of the polypeptide chain called the α-helix. It is one of the most common secondary structures. The β-helix forms a similar geometric structure. Each turn of the helix contains 3.6 amino acids. Hydrogen bonds formed between amino acids in successive turns of the helix. It completes the spiral.
The folded back of the polypeptide called sheet-sheet. This creates a folded sheet structure.
3. Tertiary structure of proteins
The spherical structure formed by bending and bending the polypeptide chain called tertiary structure. This forms the third structure (shape) of the protein.
Tertiary convection of proteins maintained by three bonds: ionic bonds, hydrogen bonds and disulfide bonds (S-S). The most stable tertiary configuration designed in an aqueous environment. Hydrophobic amino acids buried in an aqueous medium. Hydrophilic (hydrophilic) amino acids are present on the surface of protein molecules.
4. Quaternary structure of proteins
The structure formed by aggregation of tertiary polypeptide chains called quaternary structure. These polypeptide chains held together by hydrophobic interactions, hydrogen bonds, and ionic bonds. Hemoglobin carries oxygen from red blood cells.
Functions Of Protein
Proteins are the most abundant compounds present in cells. Protein accounts for more than 50% of the organism’s dry weight. They are present in all types of cells and all parts of cells. Proteins perform the following functions:

Protein
- They form many structures of cells, such as cell membranes.
- All enzymes are essentially proteins. These enzymes regulate cell metabolism.
- Most hormones are also proteins in nature. Hormones regulate metabolic processes.
- Some proteins (such as hemoglobin) can used as carriers. They transport specific substances, such as oxygen, lipids, metal ions, etc.
- Some proteins called antibodies. Antibodies can protect the body from pathogens.
- Blood coagulation proteins prevent blood loss from the body after injury.
- Proteins cause biological movement. They also cause chromosomal movement in late stages of cell division.
- They form the plasma membrane of the cell.
Proteins in plant seeds.
During oxidation, it provides energy. Proteins act as membrane receptors and transporters, from which important substances pass.
Amino Acids
Compounds with amino and carboxyl groups (COOH) and associated with the same carbon atom called alpha carbons and called “amino acids”. There are 170 types of amino acids in cells and tissues. Only 25 amino acids make up a protein. However, most proteins contain 20 amino acids. They have the common formula as amino groups.
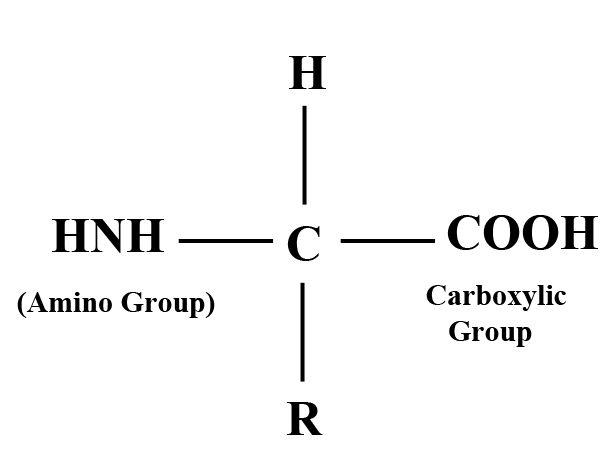
Glycine amino acids may contain hydrogen atoms. Alternatively, it may contain HCH in alanine amino acids. Or it could be some other group. Therefore, amino acids differ mainly due to the type or nature of the R group.
- Five-Kingdom System of Classification And Characteristics
- Algae: Its Characteristics and Classification
- Slime mold: Structure and Its Types