What is Respiration?
Energy is essential for the functioning of living organisms. The process of respiration provides this cellular energy. The act of breathing is universal. Respiration is the process through which cells release their greatest amount of stored energy by breaking down complicated carbon molecules.
There are two ways to use the term breathing in biology:
- External respiration refers to the process in which CO2 and O2 are released from the body and taken in from the surrounding air. The most popular phrase.
- Cellular respiration involves the slow breakdown of C chain molecules and the subsequent release of energy inside the cell.
- Carbohydrates (respiratory substrates) synthesized during photosynthesis are oxidized to carbon dioxide and oxygen is reduced to water in a sequence of enzyme-controlled oxidation-reduction processes known as respiration. The energy released by the break in the respiratory substrate is a consequence of this connection.
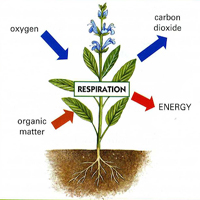
Adenosine triphosphate (ATP) molecules store the vast majority of this potential energy. Tissue respiration is another name for this process since it occurs inside the cell. Gas exchange, often known as external respiration, is the process by which organisms either take in oxygen from the atmosphere or release carbon dioxide. The steps involved may be summed up as follows:
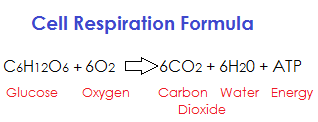
Types of Respiration
Cells primarily use glucose for energy. Gives fuel for the body’s metabolic processes. The capacity to metabolize glucose relies on the presence of oxygen. Before mitochondria develop, glucose splits into two molecules of pyruvate. Glycolysis refers to this chemical process. Ethylene glycol stands in for glucose, and cleavage is synonymous with breakdown. Hence, glycolysis denotes the division of sugar. That’s a cytoplasmic event.

All cells undergo this response. The first cell that originated on Earth may have undergone a similar response, according to biologists.
The subsequent stage of cellular respiration varies depending on the kind of cell being studied. Pyruvate may be processed in three different ways:
- Alcoholic fermentation
- Fermentation of lactic acid
- Air-breathing
The first two processes are anaerobic. In the absence of oxygen, these processes are known as anaerobic reactions. Only in the presence of oxygen, as in air respiration, are glucose molecules completely decomposed. During respiration, glucose is converted into carbon dioxide and water, producing energy.
Aerobic respiration, which needs oxygen, may take place both with and without oxygen, whereas anaerobic respiration, which does not require oxygen, can take place either with or without oxygen. Certain living things (like Clostridia) can only survive in an oxygen-free environment. Since oxygen and aerobic respiration are essential for all living things, aerobic bacteria were given this name. Breathing air produces carbon dioxide and water as waste products.
1. Anaerobic Respiration
Anaerobic respiration occurs in the absence of oxygen. It has two types:
- Certain simple cells and some eukaryotic cells, like yeast, are capable of alcoholic fermentation. In this scenario, alcohol fermentation converted pyruvic acid into ethanol and carbon dioxide.
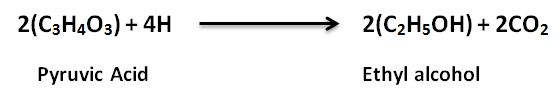
2. Lactic acid fermentation: In this case, each pyruvate molecule converted to C3H6O3 lactic acid.

Muscle cells in humans and other animals engage in this kind of anaerobic respiration. It happens in very strenuous activities like jogging. Extreme exercise prevents oxygen from reaching cells. Hence, pyruvate is broken down into lactate.
The conversion of glucose into alcohol and lactic acid releases a negligible amount of energy. Glucose only converts into ATP at a rate of 2%. (adenosine triphosphate).
2. Aerobic Respiration
It occurs in the presence of oxygen. (Read More….)
Significance of Respiration
The process of breathing is important since it serves as a source of energy. The chemical’s potential energy was released into the form of energy used by ATP.
The respiratory system’s ability to transform metabolic intermediates from the glycolysis route and the TCA cycle into useful metabolic products for the cell is perhaps its most essential feature. Amino acids are an example of a metabolic intermediate. Cell wall synthesis, nucleotide synthesis, porphyrin precursor synthesis, fatty acid synthesis, carotenoid synthesis, gibberellin synthesis, and acetic acid production all need pentose.
RESPIRATORY QUOTIENT
The respiratory ratio is the ratio of the number of moles of carbon dioxide exhaled by tissues over time to the number of moles of oxygen taken in. As a result, the response
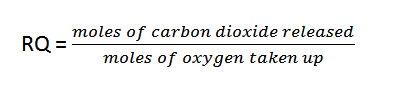
By analyzing the respiratory quotient of tissues, we may learn about the composition of the plant’s respiratory matrix.
- If glucose (carbohydrates) is a respiratory substrate, then the respiratory quotient will be:
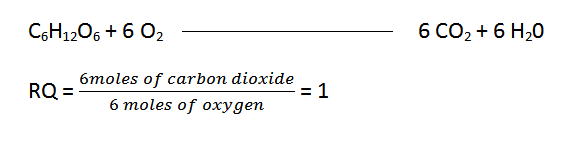
And therefore,
Glucose (carbohydrates) RQ = 1
2. As fats do not oxidize quickly, they contain extremely little oxygen. You should start by dissolving it in glycerin and fatty acids. When oxygen is used up in this process, more oxygen is required for the fat to be completely oxidized, and the reaction quality (RQ) is less than 1.

And therefore,
Fat RO = 0.7
- Protein, like fat, is not essential to the process of breathing. Prepare an amide or ammonia from them first. As a result, more oxygen is needed to thoroughly oxidize them. RO values fall between 0.79 (for amide) to 0.99. (for ammonia).
- The respiratory quotient in succulents when malic acid is the sole respiratory substrate is:
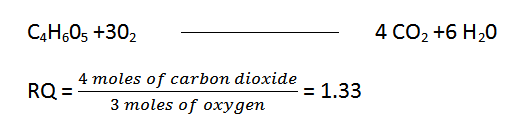
Respiratory Substrates
The term “respiratory substrates” refers to any organic vegetable component that is oxidized while breathing, either partly or totally, to carbon dioxide and water. High plant cells need heavily on carbohydrates for their respiratory needs.
Sucrose and starch are the two most common types. Sugars such as sucrose, fructose, and glucose are the most abundant forms of soluble plant nutrition. The primary carbohydrate carried by plants is also sucrose. Plants rely mostly on starch as a reserve.
Other compounds are also utilized as respiratory substrates alongside carbohydrates. Castor oil seeds, for instance, have a lot of fat stores in the endosperm tissues. The fat in these seeds germinated into sucrose, which the developing embryo then breathed in.
Examples
Organic acids may serve as respiratory substrates in some tissues. For instance, succulent plants (those with meat, generally belonging to the Crassulaceae family) store 4-C malic acid in their leaves at night, which is then oxidized in CO2 and water. Glycolic acid, another 2-carbon organic acid generated in plants exposed to brighter light, is likewise employed as a respiratory substrate.
Only under exceptional circumstances can proteins breathe. Protein breakdown was detected in extracted leaves. Protein functions as a substrate for respiration in the early stages of germination in seeds with substantial protein reserves. The protein is oxidized to carbon dioxide and water after being split into amino acids and an intermediate.
Role of Mitochondria in Respiration
Large mitochondria may take the form of granules or long filaments. In both animal and plant cells, it may be found all around the cytoplasm. The mitochondrial membranes are bi-layered. The film completely around the object. There are creases or precisely marked indentations in the inner membrane. The mitochondria are also affected by these fissures.
Cellular respiration is facilitated by mitochondria. They do this by transforming the potential energy of organic molecules into ATP. Enzymes and coenzymes are abundant in mitochondria. These enzymes and enzymes use glucose molecules to slowly release energy. Some cellular processes need this kind of energy. Hence, mitochondria may be thought of as the “powerhouse” of a cell.
Adenosine Triphosphate (ATP) and its Importance
ATP is an abbreviation for adenosine triphosphate. Discovered in every single living thing. ATP is a crucial biochemical. It is crucial in transforming the bulk of the important energy.
P is shorthand for the complete phosphate family. High-energy bonds are present in the second and third phosphate molecules. These bonds, when hydrolyzed, release more energy than those of standard ATP molecules. Around 7.3 kilocalories of energy are released at the phosphate split of the last ATP molecule. This means a lot of energy may be stored in a relatively tiny device. Constant access to ATP energy.
Many cellular activities are powered by ATP molecules. Complex molecule production, active transport across cell membranes, muscular contraction, and nerve transmission are all examples of these processes.
Biological Oxidation
Free energy is a permanent need for living things. The different redox processes are the source of this energy. Certain bacteria perform an oxidation reaction during the process of photosynthesis and chemical synthesis. That’s why they can get by without using any kind of free energy. Free energy is a prerequisite for all other interactions.
The process of oxidation during respiration provides this vitality. Hydrogen removal may occur during biological oxidation. What dehydrogenase catalyzes. a dehydrogenase paired with a select group of other enzymes. Oxidation occurs during the process of cellular respiration.
MECHANISM OF RESPIRATION
The term “respiration” refers to the multi-step process via which glucose is oxidized. There are three distinct phases to these reactions:
I. Glycolysis
A group of cytoplasmic liquid enzymes is responsible for its production (the liquid part of the cytoplasm). Glucose undergoes partial oxidation to generate two molecules of pyruvate (3C compounds), together with adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH).
2. Carboxylic acid cycle (TCA) or Krebs cycle
The glucose is converted to carbon dioxide and water in this cycle. Around 10 NADH are created in this cycle. The soluble enzyme is necessary for the TCA cycle to function in the mitochondrial matrix. Due to the oxidation of carbon to carbon dioxide, this process is sometimes referred to as oxidizing carboxyl removal.
3. The electronic transmission chain
Electronic vectors attached to mitochondrial membrane proteins make up this system. Electrons from glycolysis and the TCA cycle are used to convert NADH to oxygen. A great deal of power is lost during a wire transmission. They are mostly synthesized from ADP and Pi and stored in ATP.